$5 for a Busch Light? That makes you an idiotAt what point do you draw the line? I don’t like this and think it’s being pushed by corporations, not people. Charmin is probably upset they didn’t think of it.
What about a bar charging me $5 for a busch light? Does this count?
What about the guy charging me $2,500 to flatten my driveway of potholes?
Supply and demand. Now, the asshole running out and buying off the shelf, to overcharge the neighbor. That guy needs to be charged. He needs to pay up and do some public services to face the community he tried to screw.
Slippery slope
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Welcome to TheOhioOutdoors
Wanting to join the rest of our members? Login or sign up today!
Login / Join
2019-nCoV (Coronavirus)
- Thread starter Jackalope
- Start date
Geezer II
Bountiful Hunting Grounds Beyond.
PrayersIt made it's way to my dad's work. They sent 3 people home and all 3 were in the office yesterday. Dad is 66, my mom 61 and a for sure goner if she gets this. My wife is still working and the way they've handled it at her hospital, it's just a matter of when she's exposed. This is a terrible moment in time to say the least.
Prayers sent Jesse!
I can only hope that the current acting Ohio Health Director is seriously considering to approve some of these potential treatment options, or already on board with it. I couldn't find anything stating what the hell they are doing for the seriously ill from the corona-virus 19 yet.
IDK, perhaps someone here can shed some light with us commoners that is currently in the healthcare profession battling the problem?
An article that came out 6 days ago from Forbes with information pertaining to Hydroxychloroquie .
What Exactly Is Hydroxychloroquine, The Drug That Is Being Tested As The First Potential Coronavirus Treatment
https://www.forbes.com/sites/saibal...potential-coronavirus-treatment/#11ea8fba7994
I can only hope that the current acting Ohio Health Director is seriously considering to approve some of these potential treatment options, or already on board with it. I couldn't find anything stating what the hell they are doing for the seriously ill from the corona-virus 19 yet.
IDK, perhaps someone here can shed some light with us commoners that is currently in the healthcare profession battling the problem?
An article that came out 6 days ago from Forbes with information pertaining to Hydroxychloroquie .
What Exactly Is Hydroxychloroquine, The Drug That Is Being Tested As The First Potential Coronavirus Treatment
https://www.forbes.com/sites/saibal...potential-coronavirus-treatment/#11ea8fba7994
Sorry to hear Jesse, I hope they’re going to be okay. I hope the FDA gets this medication out there sooner rather than later. I heard on Fox somewhere 20 people who had it were given it and 19 got cured real fast and the one eventually did.
Geezer II
Bountiful Hunting Grounds Beyond.
Big_Holla
Senior Member
It made it's way to my dad's work. They sent 3 people home and all 3 were in the office yesterday. Dad is 66, my mom 61 and a for sure goner if she gets this. My wife is still working and the way they've handled it at her hospital, it's just a matter of when she's exposed. This is a terrible moment in time to say the least.
Definitely thinking of your family right now Jesse. Getting too close for comfort when it gets this close to home.
This kinda blew my mind. Now one of the weather websites are tracking the confirmed cases of Covid-19.
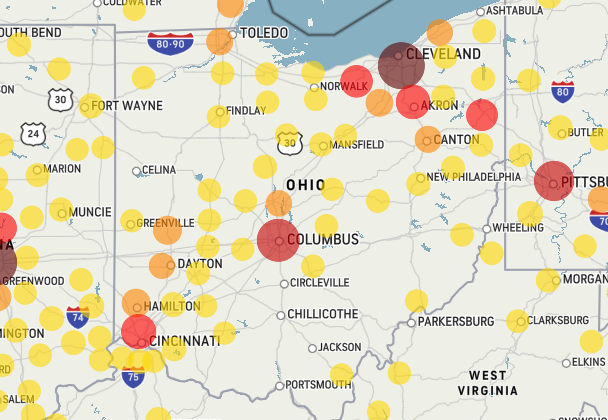
https://www.accuweather.com/en/us/national/covid-19
https://www.accuweather.com/en/us/national/covid-19
Ohiosam
*Supporting Member*
My wife's employer...
March 25, 2020

Racing Against the Clock to Combat COVID-19
Bringing a diagnostic test to market typically takes months, even years. We exceeded that by far with our new molecular test for COVID-19. In just weeks, the test was developed, approved, and out the door to hospitals and labs across the United States thanks to the incredible efforts of our colleagues in Diagnostics.
The critical first step in battling a pandemic like COVID-19 is diagnostic testing to help identify those infected to get them care and to stem the spread of the virus.
We helped answered that need with our newly developed Abbott RealTime SARS-CoV-2 molecular test, designed for use on our m2000 RealTime system. Used in more than 175 hospitals and reference labs in the United States, our m2000 is an automated system that can process hundreds of specimens in 24 hours, which is critical to helping contain the spread of COVID-19.
On March 18, we received Emergency Use Authorization (EUA) for our test from the U.S. Food and Drug Administration (FDA) and, on that same day, we immediately shipped 150,000 assays to our U.S. customers.
Typically, a test like this would take a year or more to develop and get to market, but we accomplished it in weeks, thanks to the dedication and hard work of our colleagues across Diagnostics.
“We’re so proud to have developed a coronavirus test that will help healthcare providers on the frontline of care,” said Daman Kowalski, Vice President and head of Molecular Diagnostics. “We knew that speed was critical, so we mobilized a cross-functional team quickly from Research and Development across Core Diagnostics and Molecular, Quality, Operations, Manufacturing, and Sales.”
It was a challenge that, to many, may have seemed impossible to meet. But not to us. Abbott has long been a global leader in infectious disease testing, including developing the first HIV test. Our scientists work daily to identify new or unknown pathogens and develop tests to address these new threats.
The team knew that with COVID-19, time was of the essence.
“We were running against the clock,” said Daman. “So, being able to achieve the best we could in the shortest amount of time – without compromising quality, safety, and accuracy – was key.”
And that’s exactly what we did.
“To develop the test, we put a cross-divisional team together of highly skilled scientists who have done this for many years, who knew what was needed to take something from concept and bring it to market,” said Klara Abravaya, Senior Research Fellow in Molecular.
To be most efficient, the team split up into groups, with each operating on different shifts so that testing and development would be performed at all times, 24 hours a day, seven days a week.
“We all worked together,” said Klara. “Knowing the situation, we knew we had to go much, much faster than usual. Every day we went through every single piece of data, took it one step at a time and, because of the expertise of our cross-divisional team, we were able to come up with innovative ideas for getting a high-quality test out the door as quickly as possible.”
From the onset, the team was in frequent contact with our customers to determine the needs for the test.
“We had to collaborate closely with our customers so we could understand the disease, understand what our assay design needed to be, and what we needed to deliver to the market,” said Danijela Lucic, Global Scientific Affairs Manager in Molecular. “As we would get their feedback, we would pass it along to our core research team so they could take those concepts and apply them to their test design. Then, once we outlined our design, we went back to our customers to ensure what we were thinking about for the test would meet their clinical needs.”
It was truly a team effort, with multiple parts happening simultaneously.
“We were really doing everything in parallel,” said Daman. “For example, while our scientists were developing the test, our regulatory colleagues were writing the submission and communicating with the FDA, and the manufacturing facility was making the reagents for the test. So, as soon as we received EUA, we were ready that day to ship the tests. It was one of the best cross-divisional efforts I’ve seen in all my years at Abbott.”
Chris Slachta, Divisional Vice President of Operations in Molecular, echoed that sentiment.
“We were manufacturing product and designing all in the same day,” he said. “We were going right from R&D into manufacturing, doing as many quality checks as we could to make sure the test was robust and would meet our customers’ needs.”
And working with suppliers was key, too, said Chris.
“We can’t make a test unless we have supplies,” he said. “So, we worked with our suppliers to determine how much material they could get us, how fast they could get it, and what kind of capacity they had that they could build up. And we did the same internally as well, based on our available capacity, with regards to our people, the direct and indirect labor needed, all the way through to our equipment, our filling machines, and everything else we needed to make the test so we could get it out to our customers.”
But it wasn’t just the team’s hard work that made a difference – so did their positive attitudes and determination.
“Colleagues were working extraordinary hours for two weeks straight,” said Daman. “They never complained or said ‘we can’t do this.’ Their expertise, combined with their willingness to go the extra mile for our company and all those we serve, really shows the caliber of Abbott people, our caring, our giving, and our commitment to doing the right thing.”
When all was said and done, and the test was developed, approved, and ready to go, it was a moment the team will always remember.
“It was extremely rewarding to know we developed a test that’s urgently needed to fight this pandemic,” said Klara.
Daman said, “It was such a tremendous sense of accomplishment, very humbling. I don’t think it really hit us until we started shipping the test and received so many calls and notes from our customers thanking us and saying how happy they were with the test.
“I’m so proud of our colleagues. Everyone worked tirelessly and with enthusiasm, knowing what a difference we were making. We show up in times that matter the most. Now is an unprecedented time where healthcare is at the center of this global pandemic. And we can proudly say we helped make a difference.”
March 25, 2020
Racing Against the Clock to Combat COVID-19
The critical first step in battling a pandemic like COVID-19 is diagnostic testing to help identify those infected to get them care and to stem the spread of the virus.
We helped answered that need with our newly developed Abbott RealTime SARS-CoV-2 molecular test, designed for use on our m2000 RealTime system. Used in more than 175 hospitals and reference labs in the United States, our m2000 is an automated system that can process hundreds of specimens in 24 hours, which is critical to helping contain the spread of COVID-19.
On March 18, we received Emergency Use Authorization (EUA) for our test from the U.S. Food and Drug Administration (FDA) and, on that same day, we immediately shipped 150,000 assays to our U.S. customers.
Typically, a test like this would take a year or more to develop and get to market, but we accomplished it in weeks, thanks to the dedication and hard work of our colleagues across Diagnostics.
“We’re so proud to have developed a coronavirus test that will help healthcare providers on the frontline of care,” said Daman Kowalski, Vice President and head of Molecular Diagnostics. “We knew that speed was critical, so we mobilized a cross-functional team quickly from Research and Development across Core Diagnostics and Molecular, Quality, Operations, Manufacturing, and Sales.”
It was a challenge that, to many, may have seemed impossible to meet. But not to us. Abbott has long been a global leader in infectious disease testing, including developing the first HIV test. Our scientists work daily to identify new or unknown pathogens and develop tests to address these new threats.
The team knew that with COVID-19, time was of the essence.
“We were running against the clock,” said Daman. “So, being able to achieve the best we could in the shortest amount of time – without compromising quality, safety, and accuracy – was key.”
And that’s exactly what we did.
“To develop the test, we put a cross-divisional team together of highly skilled scientists who have done this for many years, who knew what was needed to take something from concept and bring it to market,” said Klara Abravaya, Senior Research Fellow in Molecular.
To be most efficient, the team split up into groups, with each operating on different shifts so that testing and development would be performed at all times, 24 hours a day, seven days a week.
“We all worked together,” said Klara. “Knowing the situation, we knew we had to go much, much faster than usual. Every day we went through every single piece of data, took it one step at a time and, because of the expertise of our cross-divisional team, we were able to come up with innovative ideas for getting a high-quality test out the door as quickly as possible.”
From the onset, the team was in frequent contact with our customers to determine the needs for the test.
“We had to collaborate closely with our customers so we could understand the disease, understand what our assay design needed to be, and what we needed to deliver to the market,” said Danijela Lucic, Global Scientific Affairs Manager in Molecular. “As we would get their feedback, we would pass it along to our core research team so they could take those concepts and apply them to their test design. Then, once we outlined our design, we went back to our customers to ensure what we were thinking about for the test would meet their clinical needs.”
It was truly a team effort, with multiple parts happening simultaneously.
“We were really doing everything in parallel,” said Daman. “For example, while our scientists were developing the test, our regulatory colleagues were writing the submission and communicating with the FDA, and the manufacturing facility was making the reagents for the test. So, as soon as we received EUA, we were ready that day to ship the tests. It was one of the best cross-divisional efforts I’ve seen in all my years at Abbott.”
Chris Slachta, Divisional Vice President of Operations in Molecular, echoed that sentiment.
“We were manufacturing product and designing all in the same day,” he said. “We were going right from R&D into manufacturing, doing as many quality checks as we could to make sure the test was robust and would meet our customers’ needs.”
And working with suppliers was key, too, said Chris.
“We can’t make a test unless we have supplies,” he said. “So, we worked with our suppliers to determine how much material they could get us, how fast they could get it, and what kind of capacity they had that they could build up. And we did the same internally as well, based on our available capacity, with regards to our people, the direct and indirect labor needed, all the way through to our equipment, our filling machines, and everything else we needed to make the test so we could get it out to our customers.”
But it wasn’t just the team’s hard work that made a difference – so did their positive attitudes and determination.
“Colleagues were working extraordinary hours for two weeks straight,” said Daman. “They never complained or said ‘we can’t do this.’ Their expertise, combined with their willingness to go the extra mile for our company and all those we serve, really shows the caliber of Abbott people, our caring, our giving, and our commitment to doing the right thing.”
When all was said and done, and the test was developed, approved, and ready to go, it was a moment the team will always remember.
“It was extremely rewarding to know we developed a test that’s urgently needed to fight this pandemic,” said Klara.
Daman said, “It was such a tremendous sense of accomplishment, very humbling. I don’t think it really hit us until we started shipping the test and received so many calls and notes from our customers thanking us and saying how happy they were with the test.
“I’m so proud of our colleagues. Everyone worked tirelessly and with enthusiasm, knowing what a difference we were making. We show up in times that matter the most. Now is an unprecedented time where healthcare is at the center of this global pandemic. And we can proudly say we helped make a difference.”
But a young college girl showed me her butthole for a $1.$5 for a Busch Light? That makes you an idiot
Or concert, ball game, you get the point.
Came in via email and it has me scratching my head. Why is this being strictly enforced in a time of crisis? Are they concerned of EMS workers getting sick leaving a shortage? IDK...

Ambulance staffing requirements are set forth in Ohio Revised Code 4765.43. This provision requires at least one EMT, advanced EMT, or paramedic to be in the ambulance when traveling to the scene of the emergency. When transporting a patient, two EMS certified personnel must be in the ambulance. If an EMS agency is unable, due to the current crisis, to maintain this staffing requirement, the State Board of Emergency Medical, Fire and Transportation Services (EMFTS Board), pursuant to Ohio Revised Code 4765.48, has the authority to decide if the staffing violation merits filing a complaint against the EMS organization. Therefore, the EMFTS Board has the authority to review staffing violations, but it also has the authority to decline to file a complaint. As a result, the law affords EMS agencies, with appropriate oversight from the EMFTS Board, to take action as necessary to provide EMS services below minimum staffing levels if necessary under the circumstances.
The Department of Public Safety Division of EMS recommends that EMS agencies exhaust all efforts with current staffing, mutual aid agreements, and consultation with the local county Emergency Management Agency before falling below minimum staffing. Furthermore, at least one certified EMT, advanced EMT, or paramedic should be monitoring the patient during transport. The driver should also be a person who has completed training on the operation of an emergency vehicle. The EMS agency’s medical director should be notified immediately if standard transport staffing is violated. The Division of EMS should also be notified immediately in order to help assess the scope of any staffing shortages statewide.
The EMS professionals on the EMFTS Board are well aware of the potential strain this crises may impose on the EMS community, and will utilize appropriate discretion when reviewing any ambulance minimum staffing issues.

OHIO EMERGENCY MEDICAL SERVICES
EMS MINIMUM STAFFING DURING THE STATE OF EMERGENCY
Ambulance staffing requirements are set forth in Ohio Revised Code 4765.43. This provision requires at least one EMT, advanced EMT, or paramedic to be in the ambulance when traveling to the scene of the emergency. When transporting a patient, two EMS certified personnel must be in the ambulance. If an EMS agency is unable, due to the current crisis, to maintain this staffing requirement, the State Board of Emergency Medical, Fire and Transportation Services (EMFTS Board), pursuant to Ohio Revised Code 4765.48, has the authority to decide if the staffing violation merits filing a complaint against the EMS organization. Therefore, the EMFTS Board has the authority to review staffing violations, but it also has the authority to decline to file a complaint. As a result, the law affords EMS agencies, with appropriate oversight from the EMFTS Board, to take action as necessary to provide EMS services below minimum staffing levels if necessary under the circumstances.
The Department of Public Safety Division of EMS recommends that EMS agencies exhaust all efforts with current staffing, mutual aid agreements, and consultation with the local county Emergency Management Agency before falling below minimum staffing. Furthermore, at least one certified EMT, advanced EMT, or paramedic should be monitoring the patient during transport. The driver should also be a person who has completed training on the operation of an emergency vehicle. The EMS agency’s medical director should be notified immediately if standard transport staffing is violated. The Division of EMS should also be notified immediately in order to help assess the scope of any staffing shortages statewide.
The EMS professionals on the EMFTS Board are well aware of the potential strain this crises may impose on the EMS community, and will utilize appropriate discretion when reviewing any ambulance minimum staffing issues.
- 39,066
- 274
Came in via email and it has me scratching my head. Why is this being strictly enforced in a time of crisis? Are they concerned of EMS workers getting sick leaving a shortage? IDK...

OHIO EMERGENCY MEDICAL SERVICES

Robert L. Wagoner, Interim Executive Director
www.ems.ohio.gov
EMS MINIMUM STAFFING DURING THE STATE OF EMERGENCY
Ambulance staffing requirements are set forth in Ohio Revised Code 4765.43. This provision requires at least one EMT, advanced EMT, or paramedic to be in the ambulance when traveling to the scene of the emergency. When transporting a patient, two EMS certified personnel must be in the ambulance. If an EMS agency is unable, due to the current crisis, to maintain this staffing requirement, the State Board of Emergency Medical, Fire and Transportation Services (EMFTS Board), pursuant to Ohio Revised Code 4765.48, has the authority to decide if the staffing violation merits filing a complaint against the EMS organization. Therefore, the EMFTS Board has the authority to review staffing violations, but it also has the authority to decline to file a complaint. As a result, the law affords EMS agencies, with appropriate oversight from the EMFTS Board, to take action as necessary to provide EMS services below minimum staffing levels if necessary under the circumstances.
The Department of Public Safety Division of EMS recommends that EMS agencies exhaust all efforts with current staffing, mutual aid agreements, and consultation with the local county Emergency Management Agency before falling below minimum staffing. Furthermore, at least one certified EMT, advanced EMT, or paramedic should be monitoring the patient during transport. The driver should also be a person who has completed training on the operation of an emergency vehicle. The EMS agency’s medical director should be notified immediately if standard transport staffing is violated. The Division of EMS should also be notified immediately in order to help assess the scope of any staffing shortages statewide.
The EMS professionals on the EMFTS Board are well aware of the potential strain this crises may impose on the EMS community, and will utilize appropriate discretion when reviewing any ambulance minimum staffing issues.
It reads to me that some EMS employees are bitching to their union or the Ohio board about minimum staffing in the ambulance and Ohio told them to STFU.
You good nowBut a young college girl showed me her butthole for a $1.
Or concert, ball game, you get the point.
It reads to me that some EMS employees are bitching to their union or the Ohio board about minimum staffing in the ambulance and Ohio told them to STFU.
Makes sense, thanks!
- 39,066
- 274
Today, Miami healthcare providers seem to be taking an active approach using the mentioned drugs in treating patients with the virus that meet certain conditions.
What pisses me off is the inaccurate reporting. The problem isn't that Chloriquin is not FDA approved. It's been approved since October of 1949 and it's a very safe drug. It's even approved for infants, pregnant women, and women who are breastfeeding. Shit that makes it safer than benadryl. The reason they're holding it up is because it's not been FDA approved to treat Covid."
- 25,126
- 261
I've had my fill for the day.
I'll be back at it again tomorrow and share whatever is relevant.
Everyone try to have a good evening with your family.
Good night!
I'll be back at it again tomorrow and share whatever is relevant.
Everyone try to have a good evening with your family.
Good night!
- 39,066
- 274
I've had my fill for the day.
I'll be back at it again tomorrow and share whatever is relevant.
Everyone try to have a good evening with your family.
Good night!
I didn't mean you BTW. I mean the reporter failing to clarify when he said "it's not FDA approved"
